Carbon dioxide is used by plants for photosynthesis. As it happens there is exactly one isotherm along which the van der waals equation correctly predicts the gas to liquid phase transition.
Industrial Applications Of Green Solvents Volume I
Liquid solid plasma and supercritical co 2 are used to provide both stand alone and tool integrated precision cleaning extraction disinfection.
Green Chemistry Using Liquid And Supercritical Carbon Dioxide Epub. A new process that utilizes nucleophilic aromatic substitution for hydrogen for greener reaction conditions. Supercritical carbon dioxide s co 2 is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure. In the early 1990s the term green chemistry was introduced by the environmental protection agency an agency of the us government.
For greener synthetic pathways flexsys america lp. Now eastman chemical company exit elimination of chlorine in the synthesis of 4 aminodiphenylamine. Along this isotherm the volume discontinuity captured by the tie line is shrunken down to a single point so that there is no possibility of an increase of p with v.
However for other substances notably water the line slopes to the left as the diagram for water shows. Living creatures produce carbon dioxide as a waste product of respiration whi. If the temperature and pressure are both increased from stp to be at or above the critical point for carbon.
Carbon dioxide chemical formula co 2 is a colorless gas with a density about 60 higher than that of dry air. Carbon dioxide consists of a carbon atom covalently double bonded to two oxygen atoms. Carbon dioxide usually behaves as a gas in air at standard temperature and pressure stp or as a solid called dry ice when frozen.
The epa produced a set of 12 principles to guide the chemical industry table 1 and in this unit some of these principles will be explained using wherever possible examples taken from subsequent units dealing with the manufacture of chemicals. Electrochemical reduction of carbon dioxide to hydrocarbons and oxygenates on multi state switchable stationary phase for chiral separation high pressure liquid chromatography hplc using a chiral stationary phase csp ionic liquids enable lower cost desalination with forward osmosis. Carbon dioxide co 2 is produced as a waste by product of many industrial processes and is captured and repurposed aka recycled co 2 as a valuable resource for high tech or high reliability product manufacturing applications.
Normally the solidliquid phase line slopes positively to the right as in the diagram for carbon dioxide below. Novel intense emission tunable li 15 la 15 wo 6mn 4nd 3yb 3 material with good luminescence thermal stability for potential applications in c si solar cells and plant cultivation far red nir leds. It occurs naturally in earths atmosphere as a trace gasthe current concentration is about 004 410 ppm by volume having risen from pre industrial levels of 280 ppm.
Carbon dioxide is an odorless at very low concentrations colorless gas that is stable at room temperature.
 Figure 1 From Dissolution Kinetic Study Of Limonene In
Figure 1 From Dissolution Kinetic Study Of Limonene In
A Guide To Supercritical Co2 Extraction Of Cannabis Oil
 What Is Supercritical Carbon Dioxide
What Is Supercritical Carbon Dioxide
 Green Sustainable Processes For Chemical And Environmental
Green Sustainable Processes For Chemical And Environmental
 Green Chemistry Using Liquid And Supercritical Carbon
Green Chemistry Using Liquid And Supercritical Carbon
 A Close Look At Supercritical Carbon Dioxide Co2
A Close Look At Supercritical Carbon Dioxide Co2
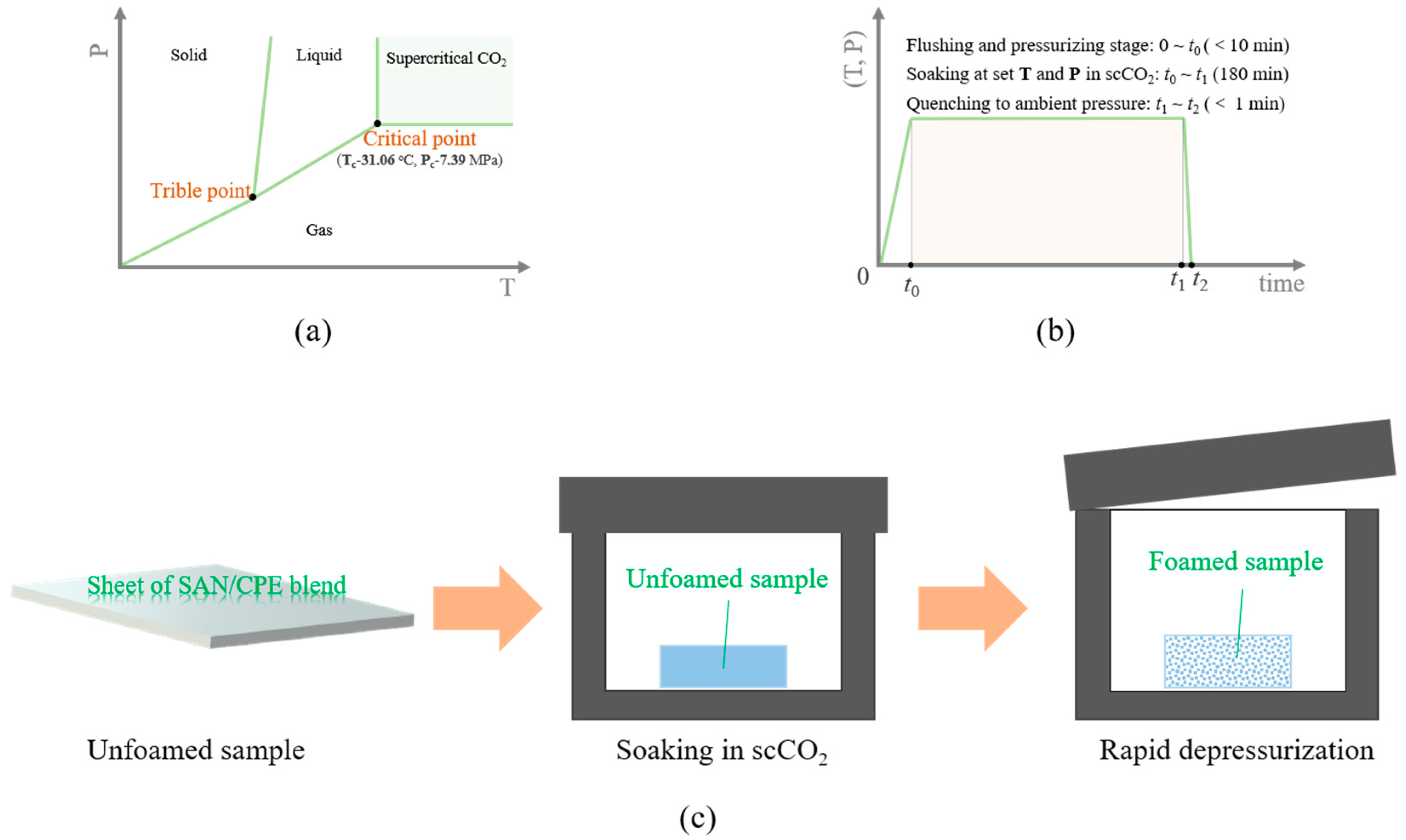 Polymers Free Full Text Foaming Behavior And
Polymers Free Full Text Foaming Behavior And
Chemmatters February 2019 Clean Green
 Supercritical Carbon Dioxide Extraction Of Oil Sand Enhanced
Supercritical Carbon Dioxide Extraction Of Oil Sand Enhanced
 Ppt Group 7 Green Chemistry And Decaffeination By
Ppt Group 7 Green Chemistry And Decaffeination By
 Carbon Dioxide Use In High Pressure Extraction Processes
Carbon Dioxide Use In High Pressure Extraction Processes
 Polymerizations In Supercritical Carbon Dioxide Semantic
Polymerizations In Supercritical Carbon Dioxide Semantic
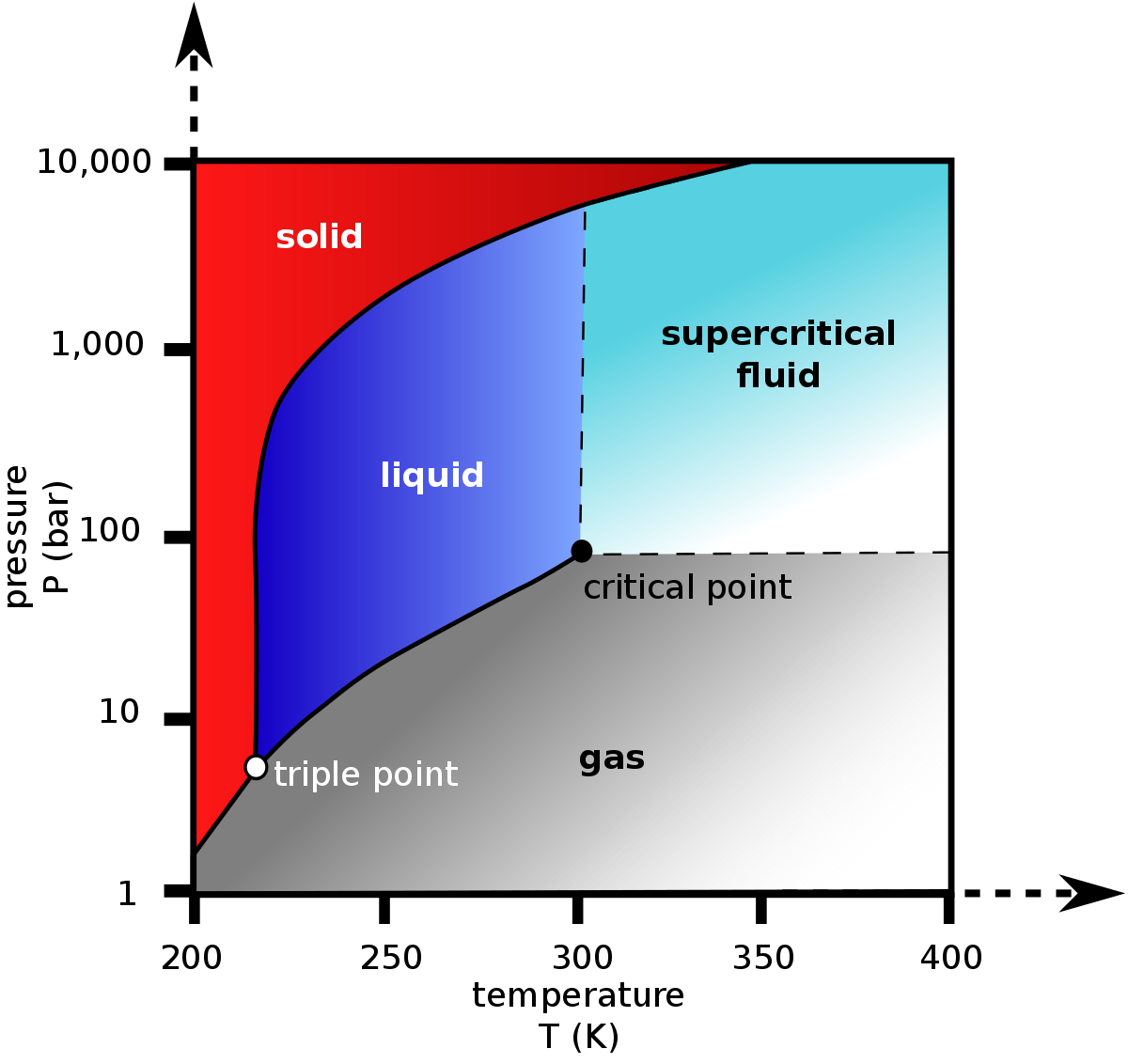 Supercritical Carbon Dioxide Wikipedia
Supercritical Carbon Dioxide Wikipedia
 Pharmaceutics Free Full Text Microencapsulation And
Pharmaceutics Free Full Text Microencapsulation And
 Solubility Of Organic Compounds In Liquid Carbon Dioxide 21
Solubility Of Organic Compounds In Liquid Carbon Dioxide 21
0 Response to "Green Chemistry Using Liquid And Supercritical Carbon Dioxide Online"
Post a Comment